Cryo-EM structure of the human mitochondrial translocase TIM22 complex
Author:Liangbo Qi, Qiang Wang, Zeyuan Guan, Yan Wu, Cuicui Shen, Sixing Hong, Jianbo Cao, Xing Zhang, Chuangye Yan, and Ping Yin
Cell Research. 08 Sep 2020. 369-372(2021)
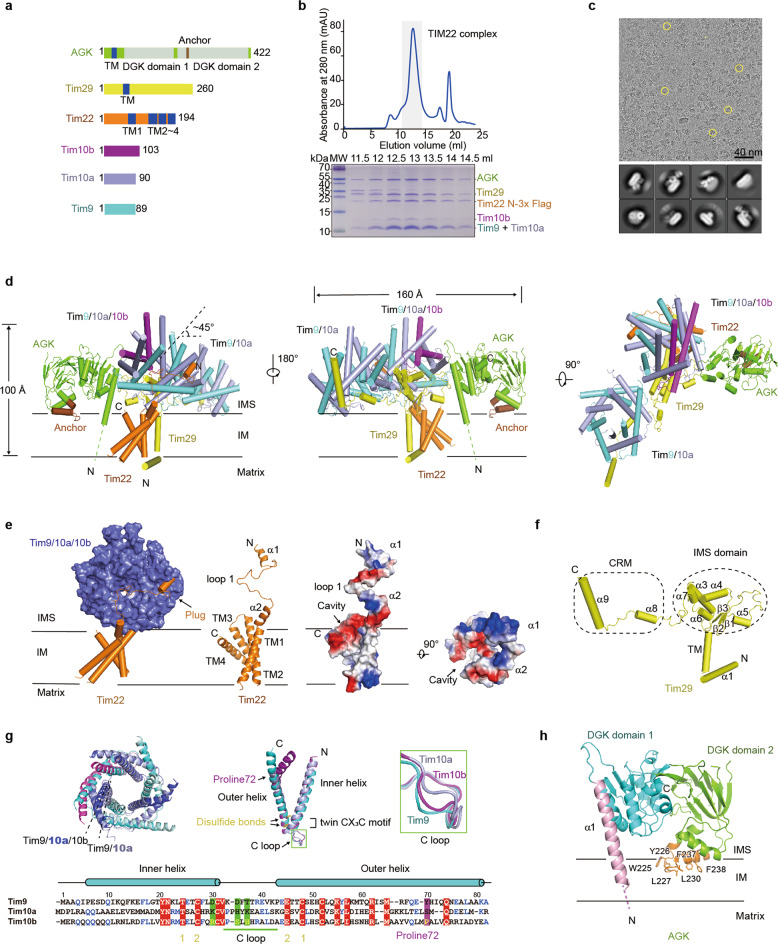
Abstract:Mitochondria are essential organelles in cellular metabolism, homeostasis, and apoptosis. Most mitochondrial proteins are synthesized as precursors in the cytosol and then imported into mitochondria by specific protein translocase complexes, including the translocase of the outer membrane complex (TOM complex), the carrier translocase of the inner membrane complex (TIM22 complex), the presequence translocase of the inner membrane complex (TIM23 complex), the sorting and assembly machinery (SAM complex), and the mitochondrial import complex (MIM complex).The TIM22 complex is responsible for the translocation and insertion of hydrophobic membrane proteins, including mitochondrial carrier proteins and translocase subunits (Tim17, Tim22 and Tim23). In humans, TIM22 is a 440-kDa complex comprising at least six components: the hypothetical channel-forming protein Tim22, three small Tim proteins (Tim9, Tim10a and Tim10b), Tim29 and acylglycerol kinase (AGK). Considering the functional importance of mitochondrial protein import, the TIM22 complex has been linked to many diseases. For example, mutations in the TIM22 gene have been reported to cause early-onset mitochondrial myopathy. AGK participates in lipid biosynthesis, and mutations in the AGK gene lead to Sengers syndrome. Mutations in the TIMM8A gene (also called DDP1) cause deafness dystonia syndrome.
Despite advances in our knowledge of the function and pathophysiology of the TIM22 complex, reports of its structural characterization are scarce. The structural studies of the TIM22 complex are restricted to the investigation of the structures of Tim9/10a and Tim9/10/12 hexameric chaperone and a nuclear magnetic resonance (NMR) analysis of carrier precursors associated with the Tim9/Tim10 complex.Here, we report the cryo-EM structure of the human TIM22 complex at an overall resolution of 3.7 Å.
doi: 10.1038/s41422-020-00400-w