Structural insight into the SAM-mediated assembly of mitochondrial TOM core complex
Author: Qiang Wang ,Zeyuan Guan ,Liangbo Qi ,Jinjin Zhang, Chen Wang, Sixing Hong ,Ling Yan, Yan Wu, Xiaoqiao Cao, Jianbo Cao, Junjie Yan, Tiantian Zou, Zhu Liu, Delin Zhang, Chuangye Yan and Ping Yin
Science. 2021 Sep 17. 373(6561)
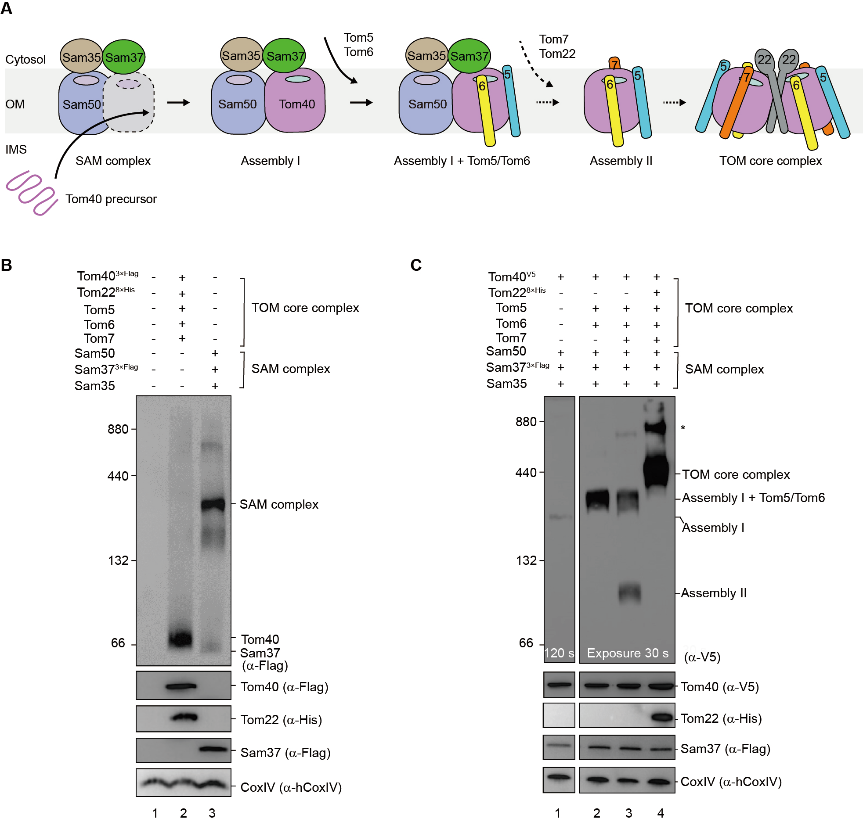
Abstract: β barrel outer membrane proteins (β-OMPs) play vital roles in mitochondria, chloroplasts, and Gram-negative bacteria. Evolutionarily conserved complexes such as the mitochondrial sorting and assembly machinery (SAM) mediate the assembly of β-OMPs. We investigated the SAM-mediated assembly of the translocase of the outer membrane (TOM) core complex. Cryo–electron microscopy structures of SAM–fully folded Tom40 and the SAM-Tom40/Tom5/Tom6 complexes at ~3-angstrom resolution reveal that Sam37 stabilizes the mature Tom40 mainly through electrostatic interactions, thus facilitating subsequent TOM assembly. These results support the β barrel switching model and provide structural insights into the assembly and release of β barrel complexes.
DOI: 10.1126/science.abh0704